
Advancements in Aluminum Alloys and Brazing for Automotive and Off-Highway Heat Exchangers

In this editorial, the article explores the significant role of aluminum alloys in heat transfer applications and delves into the advancements in material technologies, particularly aluminum alloys and brazing processes, for automotive and off-highway heat exchangers. The content covers a range of topics, from alloying elements' impact on performance to brazing techniques, fatigue resistance, and corrosion prevention. The overarching goal is to equip readers with an in-depth understanding of suitable aluminum alloys and brazing methods to meet specific heat transfer needs.
In this editorial, we will delve into the fascinating world of aluminum alloys and their crucial role in heat transfer applications. Our content will explore the functionalities of various alloying elements and their impact on the performance of aluminum alloys. We will also cover essential aspects of thermo-mechanical processing steps, highlighting their influence on the final alloy properties. Global efforts to reduce the carbon dioxide footprint and the evolving needs for smarter thermal management require continuous renewal of material technologies. The evolution of materials used, through the different alloy types, brazing operational parameters and part designs could be used to tackle these challenges. By providing in-depth analysis and data-driven insights, we aim to equip our readers with a comprehensive understanding of the most suitable aluminum alloys and brazing process for their specific heat transfer requirements.
Aluminum alloys are progressively being used for engine, transmission, charged air cooling components, electronics and battery (BEV & FCEV), and climate control systems (HVAC&R). As early as 1950, aluminum heat exchangers made moderate inroad into the automotive industry. With the introduction of the vacuum brazing technique (VAB, 1970), production of aluminum-based heat exchangers began to expand. Aluminum as material forms an oxide layer (Al₂O₃) on its surface very rapidly even at room temperature and the oxidation accelerates with increasing temperature. The oxide film is stable in aqueous solution in the pH range included between 4 and 9. Under these conditions, the aluminum is protected from corrosion. The thermodynamic potential of the Al/Al +3 is -1.67 V with respect to the normal hydrogen electrode. The oxide must be removed to be able to join aluminum parts together.
As aluminum is brazed at a higher temperature (about 600 °C) than soldering, consequently it requires better control over atmospheric oxidation of the surfaces. The oxidation can be controlled by brazing in vacuum (VAB) or controlled atmosphere (CAB). The latter involves chemicals. In addition, there is a maximum dewpoint requirement to control the humidity. The dewpoint is the temperature at which air must be cooled to become saturated with water vapor. When further cooled, the airborne water vapor will condense to form liquid water (dew). When air cools to its dew point through contact with a surface that is colder than the air, water will condense on the surface. By creating a vacuum, the amount of oxygen and humidity in the brazing chamber is minimized. In addition, aluminum alloys used in VAB must contain magnesium (Mg). Mg works as flux at high temperatures, helping to prevent surface oxidation. The dissolution of Mg2Al3 produces magnesium vapor which reacts with the oxide layer on the component surfaces and acts as a wetting agent.

Driven by the tightening of anti-pollution standards and by economic constraints, besides the New Energy Vehicle trend, the development of the automotive market has been lightening of vehicles. The development of aluminum is largely due to its lower density and specific mechanical properties (i.e., electrical and thermal conductivity relative to the density). Significant growth in the use of aluminum heat exchangers resulted from advantages of the controlled atmosphere brazing process (Nocolok process, 1978). Instead of brazing in vacuum, this process involves potassium alumina fluoride flux (flux load 2-5 g/m2), which is wetting the surface and chemically counteracting the surface oxidation. The primary role of flux is to remove oxide layer naturally present on the surface of the aluminum alloys. It also protects the bare metal from re-oxidizing in the furnace. It is expected to lower the filler metals surface tension and promote wetting of the base metal. The demand to limit oxygen content and humidity in the atmosphere is thus lower. In practice about 100 ppm oxygen in the brazing chamber can be tolerated. Brazing also requires an optimal gap width between the parts, which are to be joined together, as the brazing alloy flows with capillary action into the gaps. The max. joint gap for the CAB-process is 0.15-0.2 mm and in the VAB it is 0.05-0.10 mm. Brazing is an annealing process which means it’s crucial to understand the effect of brazing (and potential re-brazing) on the mechanical properties of the alloys (so called post-brazing properties).
Unleashing the Power of Functional Elements in Aluminum Alloys
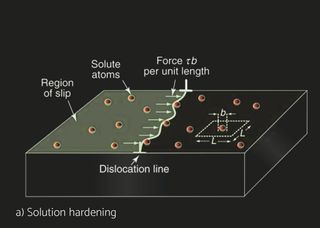
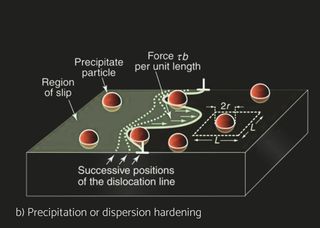
Several aluminum alloys have been developed for automotive and industrial heat exchangers. Aluminum alloys for sheet products are identified by a four-digit numerical system. The alloys are divided into eight groups based on their principal alloying element. For unalloyed wrought aluminum alloys designated 1XXX whereas the last two digits indicate the purity of the alloy. For the 2XXX to 8XXX, the second digit indicates alloy modification, whereas two last digits indicate alloy type within specific group. The characteristics of the materials differ depending on the product form and the application. Non heat treatable (NHT) and heat treatable (HT) aluminum alloys can be selected for the different components. In pure aluminum and NHT alloys, strain hardening by cold deformation increases the basic strength achieved through solid solution and dispersion hardening. Recovery and recrystallization processes during brazing, however, will eliminate any strength increase by strain hardening. HT alloys are strengthened by cold deformation as well but offer in addition the possibility of precipitation hardening. Brazing processes carried out at approx. 600°C dissolve the soluble alloying elements. Subsequent fast cooling will retain these elements in supersaturated solution. Precipitation hardening, i.e., the nucleation and growth of fine precipitates in HT alloys can then lead to a significant strength increase, both at room temperature or by ageing in the temperature range 150-200°C. For example, H14 means that alloy has been work hardened whereas H24 means partial annealing, meaning that besides work hardening, there’s an annealing cycle which reduced strength but increases ductility.

Clad metals are materials manufactured by bonding two or more different metals using a special bonding process (i.e., cladding) and have the advantage of enabling the composite use of the properties of two or more kinds of metals. A typical structure of brazing sheet consists of a core alloy which provides the strength and life cycle requirements and a clad layer which is of a low melting point Al-Si alloy. During the brazing process the clad layer melts and seals the joints between different components of the heat exchanger. The opposite side of the core alloy can be clad with sacrificial alloy Al-Zn against coolant side corrosion (i.e., 7072 alloy). Also, the flat structure of aluminum grains – achieved by certain processing steps - counteracts corrosion by directing it longitudinally (general corrosion) instead of depth (pitting corrosion). Even after recrystallization, the grains texture is elongated in the direction of rolling. This shape is sought and developed within the framework of the alloys in a concern for resistance to corrosion. Aluminum tubes are high frequency induction welded (HF), drawn or extruded. Typical clad alloys for controlled atmosphere brazing are 4343 (Si 6.8 - 8.2 %) and 4045 (Si 9.0 - 11 %), for vacuum brazing for example 4104 (besides Si, Mg 1-2%). Si lowers the melting temperature and raises the fluidity of the alloy. The aluminum silicon alloys form a binary eutectic at 11.7% silicon with a melting point of 577°C. Fin materials are typically based on the alloy 3003. There can also be more than 3 layers (clad, core, clad) and these are called multilayer clad alloys. These interlayers can be utilized to adjust the post-brazing corrosion and mechanical properties of alloys, i.e., by creating a diffusion barrier and significantly reduces silicon penetration into the core during brazing.
The typical clad ratios are determined according to product structure, joint type and brazing process requirements. For components with heavier gauge (i.e., 2 mm header plate) a clad rate of 5% would be sufficient whereas for thickness of 0.16 mm clad rate of 10% would be recommended. Components without clad require tolerance range of 0.05-0.2 mm. Components with clad can be designed close to zero tolerance. This is to optimize the capillary action which occurs because of intermolecular forces between the liquid and surrounding solid surfaces. Often comparison is made between clad 4343 vs. 4045: here the main difference is that the Si of 4343 is lower than that of 4045, the melting point of 4343 is slightly higher, and the brazing temperature is about 10℃ higher than that of 4045. In addition, the Si element content is high with 4045, and the solder fluidity is high, so the brazing quality should be considered (to avoid solder accumulation, corrosion, etc.). Also, phenomena such as Liquid Film Migration (LFM) needs to be understood and controlled.
There’re several generations of alloys where functional elements (also known as “trace” elements) have been added to enhance the technical properties of the alloys. Adding manganese (Mn) to aluminum enhances ductility, tensile strength and improves low-cycle fatigue resistance. Corrosion resistance is also measurably improved by the addition of manganese. Other alloying elements, such as titanium (Ti) and zirconium (Zr), inhibit recrystallization and allow grain structure to be controlled at elevated temperatures (grain refinement and precipitation). Copper (Cu) can be utilized as an alloying element and it has a very important role in improving the mechanical post-brazing properties: increase strength, hardness, fatigue and creep resistance. However, it can be also used to adjust the galvanic potentials (i.e., with Zn-liner) so that corrosion will occur along the side wall of the tube. The zinc is sacrificial and will “eat” along the wall: any effort for the process to make 90 degrees turn into the wall of the tube (i.e., pitting) is prevented by the cathodic charge of the core augmented by copper. Magnesium (up to 0.3-0.5%) is mainly added for solution hardening resulting in higher strength and temperature resistance. Mg is a very efficient alloying element. In fact, the most profound hardening effect beside scandium (Sc) – but Mg needs to be considered as an additive (e.g., tube and header Mg-content needs to be considered as combined).
Designations for wrought aluminium alloys
Several aluminum alloys have been developed for automotive and industrial heat exchangers. Aluminum alloys for sheet products are identified by a four-digit numerical system. The alloys are divided into eight groups based on their principal alloying element. For unalloyed wrought aluminum alloys designated 1XXX whereas the last two digits indicate the purity of the alloy. For the 2XXX to 8XXX, the second digit indicates alloy modification, whereas two last digits indicate alloy type within specific group. The characteristics of the materials differ depending on the product form and the application. Non heat treatable (NHT) and heat treatable (HT) aluminum alloys can be selected for the different components. In pure aluminum and NHT alloys, strain hardening by cold deformation increases the basic strength achieved through solid solution and dispersion hardening. Recovery and recrystallization processes during brazing, however, will eliminate any strength increase by strain hardening. HT alloys are strengthened by cold deformation as well but offer in addition the possibility of precipitation hardening. Brazing processes carried out at approx. 600°C dissolve the soluble alloying elements. Subsequent fast cooling will retain these elements in supersaturated solution. Precipitation hardening, i.e., the nucleation and growth of fine precipitates in HT alloys can then lead to a significant strength increase, both at room temperature or by ageing in the temperature range 150-200°C. For example, H14 means that alloy has been work hardened whereas H24 means partial annealing, meaning that besides work hardening, there’s an annealing cycle which reduced strength but increases ductility.
Designations for wrought aluminium alloys
Clad metals are materials manufactured by bonding two or more different metals using a special bonding process (i.e., cladding) and have the advantage of enabling the composite use of the properties of two or more kinds of metals. A typical structure of brazing sheet consists of a core alloy which provides the strength and life cycle requirements and a clad layer which is of a low melting point Al-Si alloy. During the brazing process the clad layer melts and seals the joints between different components of the heat exchanger. The opposite side of the core alloy can be clad with sacrificial alloy Al-Zn against coolant side corrosion (i.e., 7072 alloy). Also, the flat structure of aluminum grains – achieved by certain processing steps - counteracts corrosion by directing it longitudinally (general corrosion) instead of depth (pitting corrosion). Even after recrystallization, the grains texture is elongated in the direction of rolling. This shape is sought and developed within the framework of the alloys in a concern for resistance to corrosion. Aluminum tubes are high frequency induction welded (HF), drawn or extruded. Typical clad alloys for controlled atmosphere brazing are 4343 (Si 6.8 - 8.2 %) and 4045 (Si 9.0 - 11 %), for vacuum brazing for example 4104 (besides Si, Mg 1-2%). Si lowers the melting temperature and raises the fluidity of the alloy. The aluminum silicon alloys form a binary eutectic at 11.7% silicon with a melting point of 577°C. Fin materials are typically based on the alloy 3003. There can also be more than 3 layers (clad, core, clad) and these are called multilayer clad alloys. These interlayers can be utilized to adjust the post-brazing corrosion and mechanical properties of alloys, i.e., by creating a diffusion barrier and significantly reduces silicon penetration into the core during brazing.
The typical clad ratios are determined according to product structure, joint type and brazing process requirements. For components with heavier gauge (i.e., 2 mm header plate) a clad rate of 5% would be sufficient whereas for thickness of 0.16 mm clad rate of 10% would be recommended. Components without clad require tolerance range of 0.05-0.2 mm. Components with clad can be designed close to zero tolerance. This is to optimize the capillary action which occurs because of intermolecular forces between the liquid and surrounding solid surfaces. Often comparison is made between clad 4343 vs. 4045: here the main difference is that the Si of 4343 is lower than that of 4045, the melting point of 4343 is slightly higher, and the brazing temperature is about 10℃ higher than that of 4045. In addition, the Si element content is high with 4045, and the solder fluidity is high, so the brazing quality should be considered (to avoid solder accumulation, corrosion, etc.). Also, phenomena such as Liquid Film Migration (LFM) needs to be understood and controlled.
There’re several generations of alloys where functional elements (also known as “trace” elements) have been added to enhance the technical properties of the alloys. Adding manganese (Mn) to aluminum enhances ductility, tensile strength and improves low-cycle fatigue resistance. Corrosion resistance is also measurably improved by the addition of manganese. Other alloying elements, such as titanium (Ti) and zirconium (Zr), inhibit recrystallization and allow grain structure to be controlled at elevated temperatures (grain refinement and precipitation). Copper (Cu) can be utilized as an alloying element and it has a very important role in improving the mechanical post-brazing properties: increase strength, hardness, fatigue and creep resistance. However, it can be also used to adjust the galvanic potentials (i.e., with Zn-liner) so that corrosion will occur along the side wall of the tube. The zinc is sacrificial and will “eat” along the wall: any effort for the process to make 90 degrees turn into the wall of the tube (i.e., pitting) is prevented by the cathodic charge of the core augmented by copper. Magnesium (up to 0.3-0.5%) is mainly added for solution hardening resulting in higher strength and temperature resistance. Mg is a very efficient alloying element. In fact, the most profound hardening effect beside scandium (Sc) – but Mg needs to be considered as an additive (e.g., tube and header Mg-content needs to be considered as combined).


Unveiling the Secrets of CAB and VAB Processes
One of CAB’s main limitations – using standard flux – is the inability to braze materials containing more than 0.3% Mg. Such a reduction reaction of the flow efficiency during brazing leads to a formation of the compound KMgF3 whose melting temperature is 1070 ◦C, thus preventing a good flow of the filler material. In order to avoid this reaction, special flux containing cesium (Cs and atomic number 55) has been developed. These allow the brazing of material with a concentration of magnesium up to 0.5%. Cesium fluoroaluminates exist in several compositions and crystallographic states. Cs acts as a chemical scavenger for Mg. During the brazing process, cesium reacts with magnesium to form compounds such as CsMgF3. These compounds melt at lower temperatures than the filler metal. As such these compounds do not significantly interfere with aluminium brazing and allow the flux to retain much of its oxide dissolution and wetting capability. Another strategy is to prevent the magnesium to diffuse up to the surface by manufacturing alloy sheets not just three layers instead having a 4 or 5 interlayer.
Vacuum Brazing (VAB) enables to use heat-treatable alloys such as 6000-series which besides excellent strength and high corrosion resistance can be thermally treated to enhance their strength properties (capturing the full benefit of precipitation hardening). These type of heat exchangers are used for example in the aviation industry. Al-Mg-Si base alloys, which by heat treatment or aging can have higher mechanical strength than the initial production conditions. During aging / or annealing Mg and Si elements form the Mg2Si phase which precipitates in the grain boundaries and grains. This phenomenon is known as Guinier-Preston Zone meaning Mg2Si precipitation areas which do not allow dislocations to move. The peak reachable hardness by aging is dependent on the amount of Mg and Si in the alloy as well as aging temperature and time. For VAB, an additional high vacuum diffusion pump is required. Also, that means the furnace must be produced and maintained as more precise vacuum tight equipment with higher cleanliness, etc. The larger the load surface area, the larger the pumping capacity required. Because most of the magnesium vaporization occurs in the 10 -4 to 10 -5 torr range, it is the diffusion pump(s) that must handle the gas load during the mag burst with adequate backing pumps. Clean, oxide free surfaces are imperative to ensure sound brazed joints of uniform quality. Uniform capillary attraction may be obtained only when all grease, oil, dirt, and oxides have been removed from both the filler metal and the base metal before brazing. In general, the cleanliness and tolerance requirements are substantially tighter vs. CAB process.
One of CAB’s main limitations – using standard flux – is the inability to braze materials containing more than 0.3% Mg. Such a reduction reaction of the flow efficiency during brazing leads to a formation of the compound KMgF3 whose melting temperature is 1070 ◦C, thus preventing a good flow of the filler material. In order to avoid this reaction, special flux containing cesium (Cs and atomic number 55) has been developed. These allow the brazing of material with a concentration of magnesium up to 0.5%. Cesium fluoroaluminates exist in several compositions and crystallographic states. Cs acts as a chemical scavenger for Mg. During the brazing process, cesium reacts with magnesium to form compounds such as CsMgF3. These compounds melt at lower temperatures than the filler metal. As such these compounds do not significantly interfere with aluminium brazing and allow the flux to retain much of its oxide dissolution and wetting capability. Another strategy is to prevent the magnesium to diffuse up to the surface by manufacturing alloy sheets not just three layers instead having a 4 or 5 interlayer.
Vacuum Brazing (VAB) enables to use heat-treatable alloys such as 6000-series which besides excellent strength and high corrosion resistance can be thermally treated to enhance their strength properties (capturing the full benefit of precipitation hardening). These type of heat exchangers are used for example in the aviation industry. Al-Mg-Si base alloys, which by heat treatment or aging can have higher mechanical strength than the initial production conditions. During aging / or annealing Mg and Si elements form the Mg2Si phase which precipitates in the grain boundaries and grains. This phenomenon is known as Guinier-Preston Zone meaning Mg2Si precipitation areas which do not allow dislocations to move. The peak reachable hardness by aging is dependent on the amount of Mg and Si in the alloy as well as aging temperature and time. For VAB, an additional high vacuum diffusion pump is required. Also, that means the furnace must be produced and maintained as more precise vacuum tight equipment with higher cleanliness, etc. The larger the load surface area, the larger the pumping capacity required. Because most of the magnesium vaporization occurs in the 10 -4 to 10 -5 torr range, it is the diffusion pump(s) that must handle the gas load during the mag burst with adequate backing pumps. Clean, oxide free surfaces are imperative to ensure sound brazed joints of uniform quality. Uniform capillary attraction may be obtained only when all grease, oil, dirt, and oxides have been removed from both the filler metal and the base metal before brazing. In general, the cleanliness and tolerance requirements are substantially tighter vs. CAB process.
Achieving Superior Strength and Durability in Brazed Components

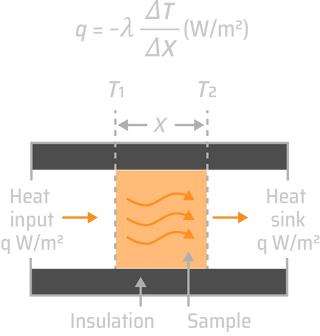
Heat is atoms or molecules in motion. In gases, they are flying between occasional collisions with each other. In solids, by contrast, they vibrate about their mean positions, the higher the temperature, the greater the amplitude of vibrations. From this perception emerges all our understanding of thermal properties of solids: their heat capacity, expansion coefficient, conductivity, even melting. Heat affects mechanical and physical properties too. As temperature rises, materials expand, the elastic modulus decreases, the strength falls and the material starts to creep, deforming slowly with time at a rate that increases as the melting point is approached until, on melting, the solid loses all stiffness and strength. As brazing is a significant heat treatment processs, its effects must be accurately identified and regulated. The depth of Sn diffusion has dependence of brazing time, temperature and diffusion coefficients. The penetration is controlled by the degree of hardening of the core material, melting point and the duration of the brazing cycle. After wetting all the free surfaces of the core material, the molten metal preferably moves towards the points of contact between the parts to be assembled. The liquid then enters the core material via its seal network of grains. The diffusion of the silicon from the liquid towards the core leads to a local decrease in the point of melting of the base alloy and thus its dissolution. Under carefully monitored braze conditions, a metallurgical bond can be formed between the parent material and the molten filler.
Brazing has many advantages when compared to other metal-joining processes. Given that brazing does not melt the base metal of the joint, it allows for more precise control of tolerances and provides a clean joint with no need for additional finishing. The meniscus (crescent shaped) formed by the filler metal in the brazed joint is ideally shaped for reducing stress concentrations and improving fatigue properties. The solidus is the highest temperature at which the metal is completely solid; the temperature at which melting starts. The liquidus is the lowest temperature at which the metal is completely liquid; the temperature at which solidification starts. Due to rapid diffusion kinetics at high brazing temperatures, the metallic bond is characterized by an interexchange of atoms between the filler and parent metal.
Oxides and other nonmetallic species are usually present on material surfaces that have been exposed to ambient atmospheres. These impurities can interfere with, or even inhibit, wetting and alloying. Aluminum forms a natural refractory oxide, that is remarkably stable, mechanically durable, and with a hardness that is inferior only to that of diamond. Moreover, the rate of oxidation roughly doubles with each 25°C rise in temperature. Thus, stable oxides can become progressively thicker and tenacious. Oxide films often endow beneficial attributes to metals, such as corrosion resistance, but their presence on braze joint surfaces present more than a nonmetallic barrier to wetting and spreading. Oxides are generally poor thermal conductors, compared with metals, and may impede heat transfer, thereby exacerbating temperature gradients and delaying fusion to the parent metal. While molten, flux forms a thermal blanket around the joint that helps to spread the heat evenly during the heating cycle. It reduces the surface tension between the joint surfaces, thereby enhancing wetting behavior. Reduction will occur when the free energy curve for metal-oxide formation lies above the oxygen partial pressure curve, at the temperature of interest. The effectiveness of using the process temperature and oxygen partial pressure to control the oxide reduction is limited further by the presence of absorbed water vapor. The desorption of water vapor increases the oxygen partial pressure in the chamber, and this has a deleterious effect on the oxide removal process. At any given temperature, the equilibrium partial pressure of oxygen in the metal oxide becomes less significant; however, the bond between the oxide and the parent metal is more substantial with respect to oxide stability. Thus, the tendency of the oxide to decompose will be greater with a reduction of atmospheric oxygen and an increase in temperature. Chemical thermodynamics can be used to determine the propensity for a metal to spontaneously oxidize, or conversely, for an oxide to disassociate. One may quantify the strength of a metal-to-oxygen chemical bond by measuring the change in the Gibbs free energy. This formation energy is sometimes referred to reciprocally as the dissociation potential of the oxide. Ellingham diagrams can be used to determine the thermodynamic stability of the surface oxides, thus predicting the equilibrium temperature between the metal oxide, reduced metal and oxygen. In response to a correctly designed braze profile, the molten filler metal and the parent metal, will result in joint formation via wetting and capillary reaction; this includes the diffusion of Si into the core alloy. Here, it is important to control the magnitude of the diffusion, in order to influence the erosion of the parent material and intermetallic phase formation.


Navigating the Fatigue Landscape of Aluminum Alloys


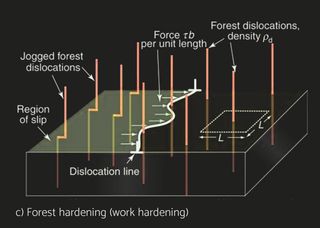
Stress causes strain. If you are human, the ability to cope with stress without undue strain is called resilience. If you are a material, it is called elastic modulus. Stress is something that is applied to a material by loading it. Strain—a change of shape—is its response; it depends on the magnitude of the stress and the way it is applied—the mode of loading. Stiffness is the resistance to change of shape that is elastic, meaning that the material returns to its original shape when the stress is removed. Strength is its resistance to permanent distortion or total failure. Stress and strain are not material properties; they describe a stimulus and a response. Stiffness (measured by the elastic modulus E, defined in a moment) and strength (measured by the elastic limit σy or tensile strength σts) are material properties. Materials are better at supporting static loads than loads that fluctuate. The long-term cyclic load a material can tolerate is barely one-third of its tensile strength (fatigue resistance). Repetition is tiring, the cause of many human mistakes and accidents. Materials, too, grow tired if repeatedly stressed, with failure as a consequence. Even when the amplitude of the cycles is very small, some energy dissipation or damping occurs. Larger amplitudes cause the slow accumulation of damage, a little on each cycle, until a critical level is reached at which a crack forms. Continued cycling causes the crack to grow until the component suddenly fails. The cyclic stress hardens the material and causes damage in the form of dislocation tangles to accumulate, from which a crack nucleates and grows until it reaches the critical size for fracture. Fatigue in materials is the result of cumulative damage processes that are usually induced by repeated loading cycles. Since the energy dissipation associated with damage is irreversible, and the loading cycles are accompanied by the evolution of heat, the corresponding relationship stress and strain is not single-valued, but rather exhibits a memory-dependence, or hysteresis.


In real operational conditions there’s of course also the variating amplitude. This problem can be addressed approximately with Miner’s rule of cumulative damage. Coined by M.A. Miner in 1945, Miners Rule is one of the most commonly used cumulative damage equations for failures caused by fatigue. Miner's rule assumes that the damage done by each stress repetition at a given stress level is equal, meaning the first stress cycle at a uniform stress level is as damaging as the last. Miner's rule operates on the hypothesis that the portion of useful fatigue life used up by a number of repeated stress cycles at a particular stress is proportional to the total number of cycles in the fatigue life, if that were the only stress level applied to the part. For example, if a part is stressed for 5,000 cycles at a stress level which would cause failure in 100,000 cycles, 5 percent of the fatigue life would be expended. Repeated stress at another stress level would consume another similarly calculated portion of the fatigue life. When 100 percent of the fatigue life is expended in this manner, the part could be expected to fail. The order in which each of these individual stress cycles is applied is not considered significant in Miner's analysis. Understanding and controlling the impact of both the internal and external loads and local stress concentrations affecting the product is the key to reliable long-life operation. Correct choice of alloys and brazing process is of an essence.
Corrosion Engineering Superheroes: Shielding Aluminum Alloys Against Corrosion
The processing from ore to metal binds energy to the metal. Thus, the metal contains more internal energy than the corresponding ore. Corrosion is a process in which this bound internal energy is trying to get released back to its lower, natural level. Simply, the metal is trying to return to its original state in nature. Thermodynamic systems proceed towards thermodynamic equilibrium – the state of maximum entropy (minimal free energy). Corrosion cannot be totally stopped but there are many ways to lower and control the rate. That area of expertise is called corrosion engineering. The general trend is an engine room built more compact, and the performance requirements for car engines increasing, resulting in elevated operating temperatures and pressures. Corrosion reactions are accelerating with increasing temperature. The continuing pressure for weight and cost reduction has resulted in strong down-gauging for the various automotive heat exchanger materials. Downgauging, on the other hand, requires an improvement of the corrosion resistance of both tube and fin alloys. The corrosion resistance of aluminum is based on an oxide film that is bonded strongly to its surface and, if damaged, it re-forms immediately in most environments. On a freshly abraded surface the oxide film is only 1 nm thick. If the destructive forces are too strong, the oxide will be hydrated faster than it is formed. Normally, relatively thick (20 to 200 nm) natural oxide films are formed on aluminum. There is no specific form of corrosion for aluminum. General corrosion, pitting, stress, intergranular and exfoliation corrosion etc. can appear when conditions are favorable for them.
Corrosion of aluminum in the passive range is localized, characterized by random formation of pits. For aluminum, pitting corrosion is mostly produced by halide ions, of which chloride (Cl-) is the most frequently encountered in service. Wrought alloys of the 3xxx series (Al-Mn and Al-Mn-Mg) have very high resistance to corrosion. Manganese is present in the aluminum solid solution, in submicroscopic particles of precipitate and in larger particles of Al6(Mn,Fe) or Al12(Mn,Fe)3Si phases, both of which have solution potentials almost the same as that of the solid solution matrix.

Then there’s so called Brown Band Phenomena (BBP). Al-Mn solid solution of the Brown Band Phenomena (BBP) has its reduced manganese concentration thus decreasing the corrosion potential of this zone, which favors the lateralization of the corrosion in the BBP (another form of sacrificial corrosion protection). Additional protection of the external surface of the tube can be achieved by using fin alloys that preferentially corrode in contact with the tube alloy. For this reason, the fin alloy must be less noble than the tube alloy. This helps in suppressing pitting of tubes. Pitting can occur in the defects (scratches etc.) of the aluminum tube surface, as the large surface oxide area is more noble than the base material inside the defect. The nobility and area differences create an electrochemical potential difference, where the less noble area will act as an anode in a corrosion cell and will be suffering from pitting corrosion. The nobility concept (galvanic differences) in aluminum core is opposite to the nobility concept of copper/brass radiator core, where tubes and solder protect catholically fins. When utilizing nobility differences for corrosion prevention, the corrosion is directed to the less important component. This type of protection, where one metal protects another one, is called cathodic protection. It works only when the more and less noble metals are in physical contact with each other and they are covered by a conductive electrolyte, usually salt containing water or water film. This way the electrical circuit becomes closed. The nobility difference of the metals and the volume and conductivity of the electrolyte determine the protection distance. In a dense radiator core, it often works between fins and tubes, as they are close enough to each other. The preferred option for achieving a fin that protects the tube is the addition of zinc, typically 1.0 to 2.5 wt. % Zn will reduce the nobility of the fin material enough. The low melting point Al-Si alloys which are used to clad the tubes for joining purpose are after brazing already slightly less noble to most of the Al-Mn based tube alloys.
The processing from ore to metal binds energy to the metal. Thus, the metal contains more internal energy than the corresponding ore. Corrosion is a process in which this bound internal energy is trying to get released back to its lower, natural level. Simply, the metal is trying to return to its original state in nature. Thermodynamic systems proceed towards thermodynamic equilibrium – the state of maximum entropy (minimal free energy). Corrosion cannot be totally stopped but there are many ways to lower and control the rate. That area of expertise is called corrosion engineering. The general trend is an engine room built more compact, and the performance requirements for car engines increasing, resulting in elevated operating temperatures and pressures. Corrosion reactions are accelerating with increasing temperature. The continuing pressure for weight and cost reduction has resulted in strong down-gauging for the various automotive heat exchanger materials. Downgauging, on the other hand, requires an improvement of the corrosion resistance of both tube and fin alloys. The corrosion resistance of aluminum is based on an oxide film that is bonded strongly to its surface and, if damaged, it re-forms immediately in most environments. On a freshly abraded surface the oxide film is only 1 nm thick. If the destructive forces are too strong, the oxide will be hydrated faster than it is formed. Normally, relatively thick (20 to 200 nm) natural oxide films are formed on aluminum. There is no specific form of corrosion for aluminum. General corrosion, pitting, stress, intergranular and exfoliation corrosion etc. can appear when conditions are favorable for them.
Corrosion of aluminum in the passive range is localized, characterized by random formation of pits. For aluminum, pitting corrosion is mostly produced by halide ions, of which chloride (Cl-) is the most frequently encountered in service. Wrought alloys of the 3xxx series (Al-Mn and Al-Mn-Mg) have very high resistance to corrosion. Manganese is present in the aluminum solid solution, in submicroscopic particles of precipitate and in larger particles of Al6(Mn,Fe) or Al12(Mn,Fe)3Si phases, both of which have solution potentials almost the same as that of the solid solution matrix.
Then there’s so called Brown Band Phenomena (BBP). Al-Mn solid solution of the Brown Band Phenomena (BBP) has its reduced manganese concentration thus decreasing the corrosion potential of this zone, which favors the lateralization of the corrosion in the BBP (another form of sacrificial corrosion protection). Additional protection of the external surface of the tube can be achieved by using fin alloys that preferentially corrode in contact with the tube alloy. For this reason, the fin alloy must be less noble than the tube alloy. This helps in suppressing pitting of tubes. Pitting can occur in the defects (scratches etc.) of the aluminum tube surface, as the large surface oxide area is more noble than the base material inside the defect. The nobility and area differences create an electrochemical potential difference, where the less noble area will act as an anode in a corrosion cell and will be suffering from pitting corrosion. The nobility concept (galvanic differences) in aluminum core is opposite to the nobility concept of copper/brass radiator core, where tubes and solder protect catholically fins. When utilizing nobility differences for corrosion prevention, the corrosion is directed to the less important component. This type of protection, where one metal protects another one, is called cathodic protection. It works only when the more and less noble metals are in physical contact with each other and they are covered by a conductive electrolyte, usually salt containing water or water film. This way the electrical circuit becomes closed. The nobility difference of the metals and the volume and conductivity of the electrolyte determine the protection distance. In a dense radiator core, it often works between fins and tubes, as they are close enough to each other. The preferred option for achieving a fin that protects the tube is the addition of zinc, typically 1.0 to 2.5 wt. % Zn will reduce the nobility of the fin material enough. The low melting point Al-Si alloys which are used to clad the tubes for joining purpose are after brazing already slightly less noble to most of the Al-Mn based tube alloys.


The Future of Thermal Management: Innovations in Automotive and Off-Highway Heat Exchanger Material Technology
Technology development and aluminum alloy development continues as new applications and requirements emerge (i.e., BEV and FCEV thermal management systems), as well as the continuous focus on topics like lightweighting the transportation, energy-efficient and sustainable manufacturing processes, and enhancing material properties for various industries. Innovation potential certainly involves developing new high-strength alloys, corrosion-resistant variants, with increasing focus on recycling and circular economy initiatives. Developing ways to use materials more efficiently is a prerequisite for a sustainable future.
Heat exchanger manufacturers should explore the development opportunities and match the material to design requirements. The process is one of narrowing the materials search space by screening out materials that cannot meet the design requirements, ranking those that remain for identifying the most promising choice. The material decisions do not end with the establishment of production. Products fail in service, and failures contain information. This information is then feed back into D/PFMEA.
On the brazing front, there’s also developments such as integrating so-called medium-vacuum purging to the CAB. This reduces nitrogen consumption significantly due to the need of single backfilling instead of several exchanges in the purging chambers. Despite the reduced N2 consumption, the brazing atmosphere purity is higher across the whole heating, brazing and initial cooling process, usually on the level of 10-20 ppm of oxygen. This provides the highest quality of brazing atmosphere for forming the joints and excellent look of the cores after brazing.**
Technology development and aluminum alloy development continues as new applications and requirements emerge (i.e., BEV and FCEV thermal management systems), as well as the continuous focus on topics like lightweighting the transportation, energy-efficient and sustainable manufacturing processes, and enhancing material properties for various industries. Innovation potential certainly involves developing new high-strength alloys, corrosion-resistant variants, with increasing focus on recycling and circular economy initiatives. Developing ways to use materials more efficiently is a prerequisite for a sustainable future.
Heat exchanger manufacturers should explore the development opportunities and match the material to design requirements. The process is one of narrowing the materials search space by screening out materials that cannot meet the design requirements, ranking those that remain for identifying the most promising choice. The material decisions do not end with the establishment of production. Products fail in service, and failures contain information. This information is then feed back into D/PFMEA.
On the brazing front, there’s also developments such as integrating so-called medium-vacuum purging to the CAB. This reduces nitrogen consumption significantly due to the need of single backfilling instead of several exchanges in the purging chambers. Despite the reduced N2 consumption, the brazing atmosphere purity is higher across the whole heating, brazing and initial cooling process, usually on the level of 10-20 ppm of oxygen. This provides the highest quality of brazing atmosphere for forming the joints and excellent look of the cores after brazing.**